A Killorglin father says the HSE’s decision to approve reimbursement for a gene therapy drug will change his son’s life.
Five-month-old Theo Whelan has been diagnosed with spinal muscular atrophy (SMA), which affects both the central and peripheral nervous systems, and voluntary muscle movement. A reimbursement scheme for the drug Zolgensma has now been approved by the HSE.
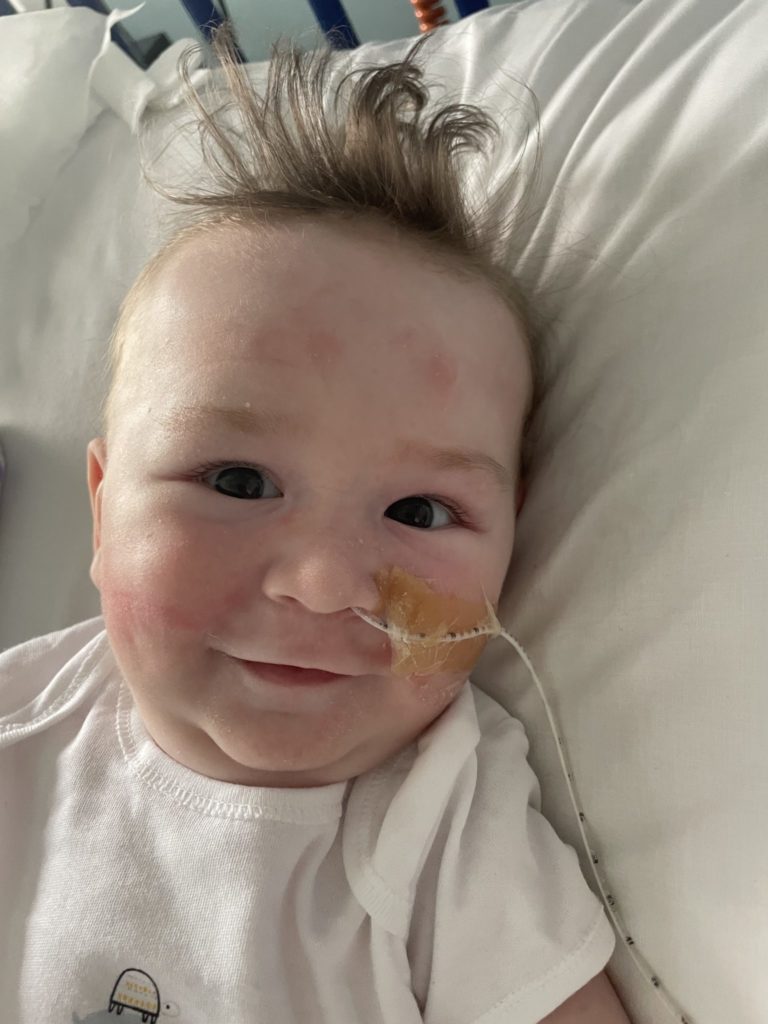
Theo’s family had been campaigning to get the drug approved, saying it would greatly improve their son’s quality of life.
His father Shane says the community’s support in recent weeks kept the family going.

Kerry Independent TD Michael Healy-Rae says that, having first raised the matter in the Dáil, he’s welcoming today’s decision.
He says the drug is a lifesaver for Theo and others affected by SMA and will give them a fighting chance.
Deputy Healy-Rae is thanking the government, the HSE and the Minister for Health Stephen Donnelly for their co-operation and understanding.
Minister for Education Norma Foley and Fine Gael TD Brendan Griffin have also welcomed the HSE decision.
SMA Ireland, the patient representative group for people affected by Spinal Muscular Atrophy, is welcoming the announcement of a pricing agreement for Zolgensma.
Director of SMA Ireland, Jonathan O’Grady says this is a hugely significant day for Irish babies with SMA, as well as their families, carers, and friends.
He says to ensure this life changing medicine will have maximum effect, it’s vital it’s administered as early as possible.
He’s therefore calling for SMA to be added to the national list of diseases assessed as part of the neonatal screening process, as is the case in many other countries.





